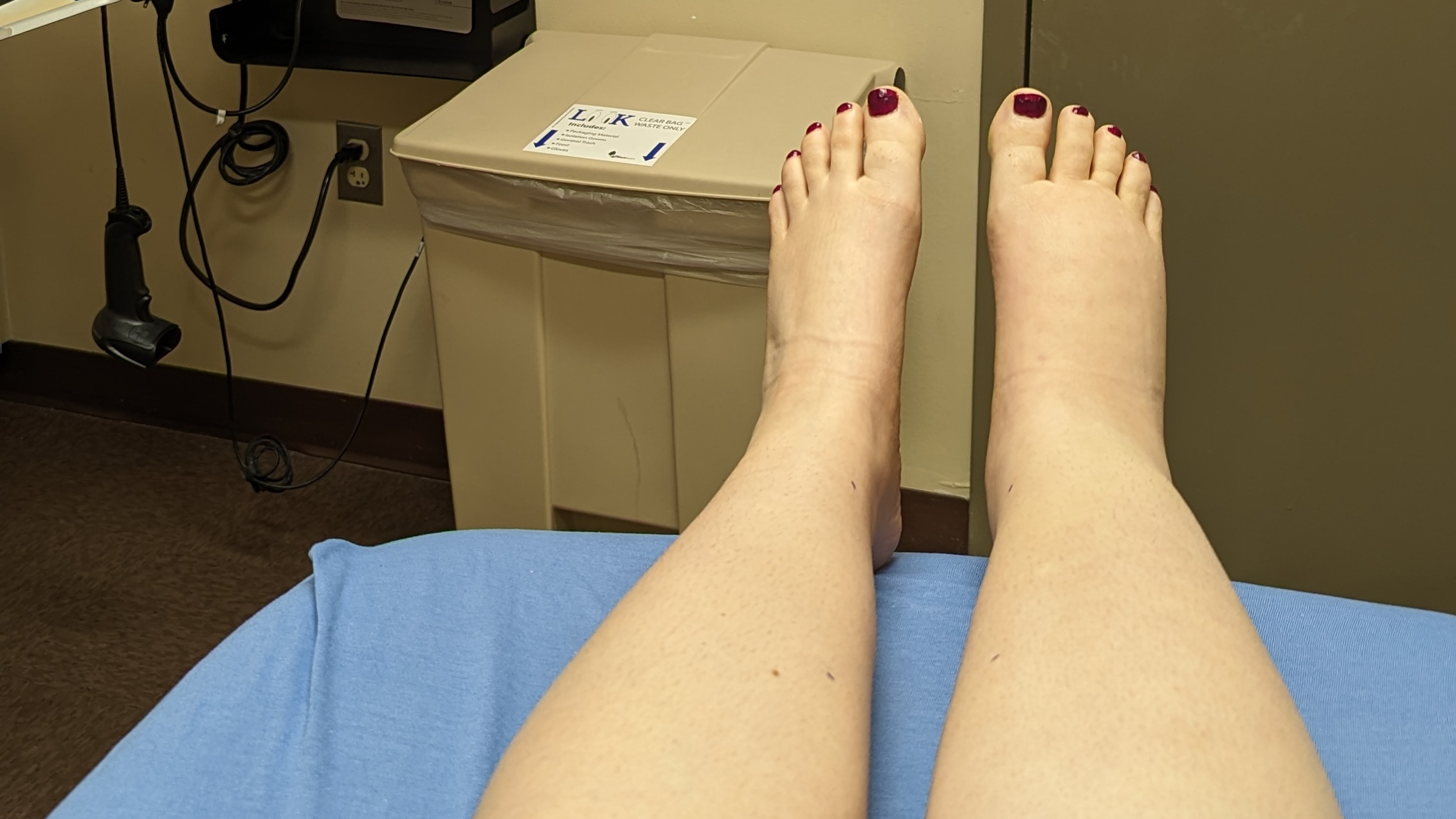
Last week I posted about a recent study that carries huge implications for lymphedema treatment and care.
The study, “The Cutaneous, Net Clinical, and Economic Benefits of Advanced Pneumatic Compression Devices in Patients with Lymphedema,” presented evidence-based research regarding the benefits of using an advanced pneumatic compression device as an at-home treatment for lymphedema. The findings include a nearly 80% reduction in the rate of cellulitis episodes within a year of use, as well as a significant reduction in the rate of hospital and physical therapy visits. Needless to say, the significance of this study is huge in terms of reducing the financial burden of lymphedema care, as well as improving the quality of life of those of us living with it.

Just a few days after the study’s publication, I had the opportunity to speak with one of the authors, Dr. Alan T. Hirsch, M.D., about the study’s impact on lymphedema treatment, insurance coverage, and the future of lymphedema research.
To speak with someone whose work could influence the way I and millions of others live with and receive treatment for lymphedema was nothing short of an inspiring experience, especially as he is genuinely invested in patient support and access to care.
To provide a little background: Dr. Hirsch is the Director of the Vascular Medicine Program at the University of Minnesota Medical School. He has treated patients with lymphedema and has designed vascular clinical research investigations as a full time University faculty member for the past twenty-five years. Recently, he has also worked as Chief Medical Officer of Tactile Medical, a company that manufactures products used to treat lymphedema and chronic venous disease. In this role, he helps design and direct lymphedema research studies. (It is important for readers to note that the relationship between his University academic and Tactile research work has been reviewed and managed by the University of Minnesota in concordance with its conflict of interest policies.) His thirty-year medical career is in the field of vascular medicine, and helping people with lymphatic, artery, or vein diseases gain access to treatment.
“I’m like you—I get very passionate,” Dr. Hirsch warned at the beginning of the conversation. “So let’s be careful! We could talk for hours about any one question!”
The Great Compression Device Debate
One of the first things I asked Dr. Hirsch was a question I get from my readers all the time: are compression devices dangerous? Could they push the swelling from the affected limb to other parts of the body?
The fear of damaging our affected limb or causing the swelling to spread is a common concern amongst us lymphies, especially as there is not much research out there to supply any definitive answers; we don’t want to make things worse! Dr. Hirsch recognizes this, saying that through most of his thirty year career there has been a “relative paucity of large high-quality studies in lymphedema.” He explained that everybody suffers in this absence of information—from patients to families to doctors, and even the government—because in the absence of information we create innuendo, rumor, and fear, eschewing treatments that could in fact help us.
“Isn’t it frustrating not to know what to believe,” Dr. Hirsch asked, “and to have to consider adding worry about a treatment on top of the worry we already have for the illness?”
“I have never known of any high quality data—in contrast to opinion or anecdote—that has really clarified objectively that damage or danger is caused by use of any form of compression,” he continued. “Where there is a medical concern, one does not idly sit and worry—worry is not a treatment. Better, one would use this concern to design a study to answer the question. Then we would know, with some certainty, of the relative benefit or harm of any compressive treatment.”
During the course of the recent national study designed by investigators from the University of Minnesota, Vanderbilt University, and Stanford University, the benefits of a Flexitouch System, an advanced pneumatic compression device designed for at-home treatment, was studied to evaluate its impact on clinical events and costs. It is important to note that these were not patients in the care of the researchers, but rather a population of over seven hundred people who received care from inumerable physicians and therapists from across the United States. The overall health impact of Flexitouch use was assessed by measuring the kinds of care that were needed by these patients in both outpatient and inpatient care settings. The result? There was no data that demonstrated transporting fluid from the limb into the central circulation via use of a pneumatic compression device caused anything adverse: there were no increases in doctor visits or infections, and no increase in healthcare utilization or cost.
“That’s good news!” Dr. Hirsch said. “There’s no known downside, defined as a measurable health risk, associated with such treatment.”
Dr. Hirsch said it was “a very positive trial,” one that provides him as a clinician with a fresh reassurance: “When my patients come see me and say that somebody told them devices are dangerous, I would say, ‘Well, no, not necessarily so. Most people benefit.’”
Dr. Hirsch made it clear, however, that pneumatic compression devices are not—in isolation—the be-all, end-all of ideal lymphedema treatment and care. Patients with lymphedema, he stated, need a comprehensive lifetime care plan, but advanced pneumatic compression devices can—and perhaps should—play a role in these care plans: “To me, the definition of good lymphedema care is a superb support system, a dedicated physical therapist or vascular clinician, and some form of effective home-based compression. These data demonstrate that this can be achieved with a good advanced pneumatic compressive device.”
The Study’s Impact on Insurance Coverage
The findings of this study also have huge implications for reducing the overall cost of care for lymphedema patients. This study demonstrated quite definitively that when the adverse events are prevented, health care costs are lowered. That is in everyone’s best interest, as episodes of cellulitis or serious skin infections hurt the patient, disrupt the family, impair work and hobbies, and are extremely expensive. Each preventable episode also likely further damages the lymphatic vessels and the skin.
Thus, access to therapies and devices that prevent skin infection are as important to the patient with lymphedema—and to society—as access to immunizations is to the health of children. Yet, achieving such success hinges on a patient’s ability to obtain an advanced pneumatic compression device for their own home treatment use, which can be difficult if their insurance creates very long delays in fostering access to an effective treatment, or simply does not cover the cost at all. I wanted to know Dr. Hirsch’s thoughts regarding insurance coverage of lymphedema treatment, and how it could change with new research such as the data supported by his study.
Considering that this new study was conducted to evaluate a large, nation-wide group of unselected patients with no potential intrinsic treatment bias, that the study was conducted by world class lymphatic disease investigators, and was published in one of the most prestigious biomedical journals, Dr. Hirsch is hopeful that the data will help assure that more access will be available for all individuals with lymphedema who desire such care. Dr. Hirsch believes this particular study could be instrumental in lowering barriers to treatment for these advanced devices and perhaps for all effective lymphedema therapies. He noted that the use of advanced pneumatic compression devices, in many ways, is also a marker of patient access to generally good care and treatment overall.
“Establishing the lymphedema diagnosis promptly, assuring that the patient is offered accurate, empowering health information, and establishing any effective lymphedema treatment improves individual health, preserves a person’s quality of life, and now is proven to do so at lower cost,” Dr. Hirsch stated. “In other words, this study clarifies that for lymphedema—like all other chronic diseases—the most costly thing for the patient and society usually is doing nothing.”
Dr. Hirsch acknowledged the preference of most health professionals and patients to live in a world in which access to all forms of treatment were “facilitated and not impaired.” For lymphedema, that would translate to prompt universal access to diagnostic examinations, to trained physiotherapists, and also to pneumatic compression devices—treatments that are effective in controlling edema, improving quality of life, lowering rates of cellulitis and hospitalization, and that amazingly are not currently universally accessible for all patients.
Lymphedema is far too often overlooked as a public health concern, but Dr. Hirsch insists that treatment access for lymphedema patients would have a beneficial effect on communities as a whole via achievement of the “Triple Aim” framework, wherein a good treatment not only benefits the individual but supports population health and lowers per capita costs of healthcare. Examples of “Triple Aim” are immunizations, prenatal care, and good nutrition for children—all things that benefit the common good.
The four authors of the study are emblematic of this multi-faceted approach to public health, as they come from four different perspectives: Dr. Hirsch, a vascular medicine specialist and population scientist; Dr. Pinar Karaca-Mandic, a health economist; Dr. Sheila H. Ridner, an academic nurse; and Dr. Stanley G. Rockson, a lymphologist and cardiologist. Together, they were able to provide a unique focus to the study that considered a bigger, more inclusive picture of the clinical challenge we all face, using data, and providing these data freely in their publication so that we all know what it means.
The Future of Lymphedema Research
By performing a large population-based research study, the authors hoped to elevate the standard of lymphedema research itself; to inspire other investigators to design other future large lymphedema trials; and for patients to volunteer and participate in them.
“We did this with the hope that we would accomplish something unusual,” Dr. Hirsch explained, “which is to use an extremely large data set and look at the health of the population, not merely the health of twenty patients in my practice or sixty patients at three medical centers.” Those smaller studies are always important, but there are so many more people “at risk” with lymphedema.
Despite this recent study, Dr. Hirsch acknowledges that lymphedema research is not at the level where it should be: “For it to be 2015, where a study of seven hundred plus patients is landmark, should not be the endgame of our research enterprise. We should be studying this disease across nations, in inherited, cancer-related, and infectious disease populations, and across the globe to prevent lymphedema. We should invest in this research to ensure that each and every treatment is understood fully for its risk and benefit—and obviously mostly to achieve real benefit.”
In order for research to continue moving forward, everyone—patients as individuals, patient advocacy groups, national research agencies, and manufacturers—need to invest in supporting the research so that we can get clear-cut answers. Without our engagement, Dr. Hirsch points out that we as a community will remain “disorganized, unmotivated, and more committed to controversy than to evidence.” This sort of complacency will not help us; we need to be proactive and keep advocating for more high quality research, and for more answers.
“For most questions that persist in healthcare, and that create undue anxiety, clear-cut answers aren’t possible if you don’t do the clinical research,” Dr. Hirsch emphasized. “The four of us [authors] hope that there will be many other studies like this to answer any particular question that Alexa and her readers think are important.”
At this point in the conversation, Dr. Hirsch asked me a question: “If you could design one research study to answer a pressing question in lymphatic disease, what would that be? What would you have us do next?”
I wasn’t sure how to answer that, except to say that, of course, a cure would be great. Prevention of the whole disease is hard because there are so many different causes, but better treatment and care are certainly possible.
“The whole point of talking to each other is so that there will be an interchange where each person’s spark helps ignite the other person’s creative spark, which helps the first person feel heard and for both people to light a fire of intense creativity,” Dr. Hirsch said. “For that is the purpose of research: to help create a better world. If we wanted to take this investigation, or any other research study in lymphatic disease, and ask experts ‘what’s the next thing to do?’, well I think that really you shouldn’t be asking me this question—we should be asking your readers.”
One way to do this, Dr. Hirsch said, is through support and participation in projects sponsored by a group called “PCORI,” the Patient-Centered Outcomes Research Institute. It’s a public-private partnership in the government and a scientific agency funded by Congress, which supports and funds patient-centered research. (See below for more information on PCORI and how to get involved.)
“The idea behind PCORI is that doctors don’t always ask the right questions. It is very often true that the people who know best about the disease are the patients, individuals, and groups affected by the health challenge,” Dr. Hirsch explained. “Great scientific research can be chartered when we recognize the value of empowerment.”
Aside from getting involved with PCORI (which Dr. Hirsch highly encourages, as do I!), what can we as patients do? We can continue having conversations about lymphedema and lymphedema awareness, for a start. Many of us don’t read medical journals, so we aren’t aware of what’s going on in the world of research and treatment: “We could publish in any journal and it will have no impact on the people for whom the research was done,” Dr. Hirsch stressed. “Only by you reblogging each major new insight is there any hope for progress to be sustained. We need to feel some optimism and to encourage each other.”
By reblogging, reposting, retweeting, sharing, speaking… there is hope. Through starting conversations and dialogues, we are bringing lymphedema—and ourselves—to the forefront and creating awareness, and through awareness there is hope for answers and for change.
“You play a role, and I play a role—we just do it from different perspectives,” Dr. Hirsch stated towards the end of our conversation. “We all need to be effective cheerleaders, to inspire each other, and to reach for evidence. Great evidence is a solid anchor for us all to feel optimism.” ♦
PCORI
The Patient-Centered Outcomes Research Institute (PCORI) is an independent nonprofit, nongovernmental organization located in Washington, DC.
Authorized by Congress in 2010, PCORI funds comparative clinical effectiveness research as well as support work that will improve the methods used to conduct such studies. Their mission is to “help people make informed healthcare decisions, and improve healthcare delivery and outcomes, by producing and promoting high-integrity, evidence-based information that comes from research guided by patients, caregivers, and the broader healthcare community.”
For more information on how to get involved with PCORI and to suggest a patient-centered research question about lymphedema, click here.



Leave a Reply